By Richard Lamar, PhD
Director of Humic Research
Bio Huma Netics, Inc.

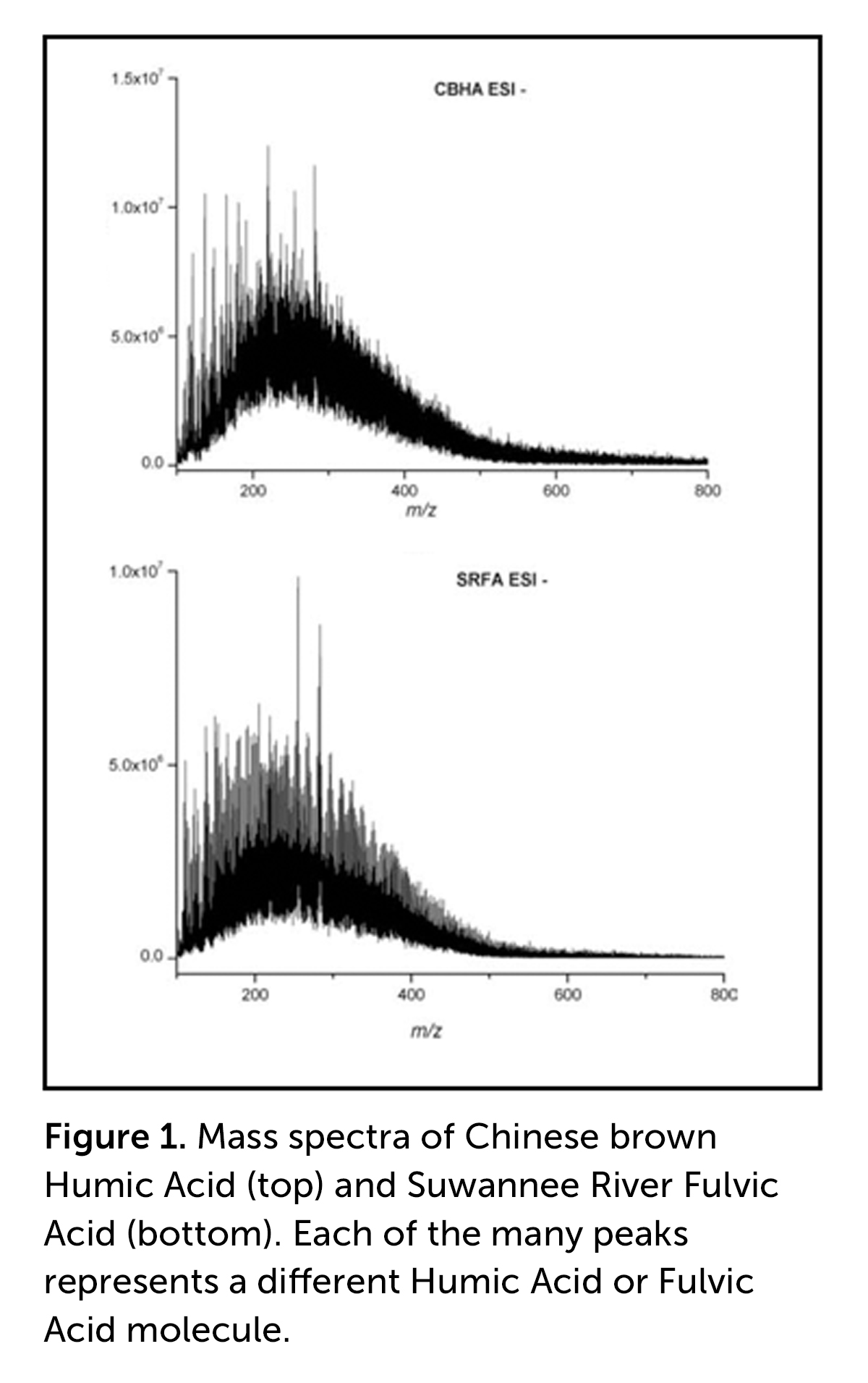
To review, the operational definitions of HA and FA are that FA are soluble in water under all pH conditions, while HA are soluble in water only under alkaline conditions. Thus, in a strong alkaline extract, such as Huma Pro® 16 (with a pH of 11.0–12.0), both HA and FA are soluble, primarily because they become salts (e.g., potassium salts), are fully negatively charged, and the negatively charged molecules separate and repel each other. If the pH is decreased to pH 1, for example with concentrated hydrochloric acid (HCl), all the COOH and Ar-OH groups become re-protonated (i.e., an H atom is added to the negatively charged COO– and Ar-O– groups) and the HA precipitates because there are no longer any negative charges to repel HA molecules, and it is no longer water soluble.
FA molecules, which possess abundant COOH and Ar-OH, as well as other oxygen-containing functional groups, remain in solution because the presence of all these groups makes H-bonding with H2O possible. Conversely, HA molecules, which possess limited numbers of oxygen-containing functional groups that do not possess enough force via H-bonding compared with the size of the molecules to keep the molecules soluble, become more hydrophobic (i.e., H2O repelling). As a result, HA molecules start to form hydrophobic aggregates, which ultimately results in their precipitation. So, when Huma Pro® 16 is added to a highly acidic fertilizer (e.g., Super Phos®), the HA precipitates and is likely to clog spray nozzles. [See our video on Mixing Liquid Humic Acids with Agrochemicals.]
The take-home message is that HA do differ from FA, but not because of their relative molecular size. They primarily differ because FA molecules contain higher numbers of oxygen-containing functional groups, which allow them, through hydrogen bonding, to remain water soluble even at strongly acidic pH values.
Related Posts

Bio Huma Netics Hosts 2016 World Conference
Bio Huma Netics, Inc. (BHN), hosted its 2016 World Conference on October 25–28 in the cities of Mesa and Gilbert, Arizona. This edition of the biannual Conference had over 150 attendees from 20 countries representing distributors, customers, and staff from BHN’s product lines of Huma Gro®, Huma Gro® Turf, and Probiotic Solutions®.

Discover Three Products to Prep Your Soil for Spring Planting
As I begin to write, I’m reminded of an excerpt from Ode to the West Wind — “If winter comes, can spring be far behind?” Well, spring is certainly not far behind, especially for those of us working in the agriculture sector. Winter can be a tricky time for growers, as many of them face

Huma Gro® Fertilizer Products Increase Cucumber Yields at ROI of 113%
The Huma Gro® fertilizer treatment increased the cucumber yield by 2% (8 bushels per acre) over the Check, with a return on investment (ROI) of 113%.

