By Richard Lamar, PhD
Senior Director of Humic Research
Bio Huma Netics, Inc.

All substances, solid AND liquid, have a chemical makeup. An acid is a chemical that can donate a proton (H+) to a water molecule (H2O, which would form H3O+) or to another chemical such as ammonia (NH3, which would form NH4+).
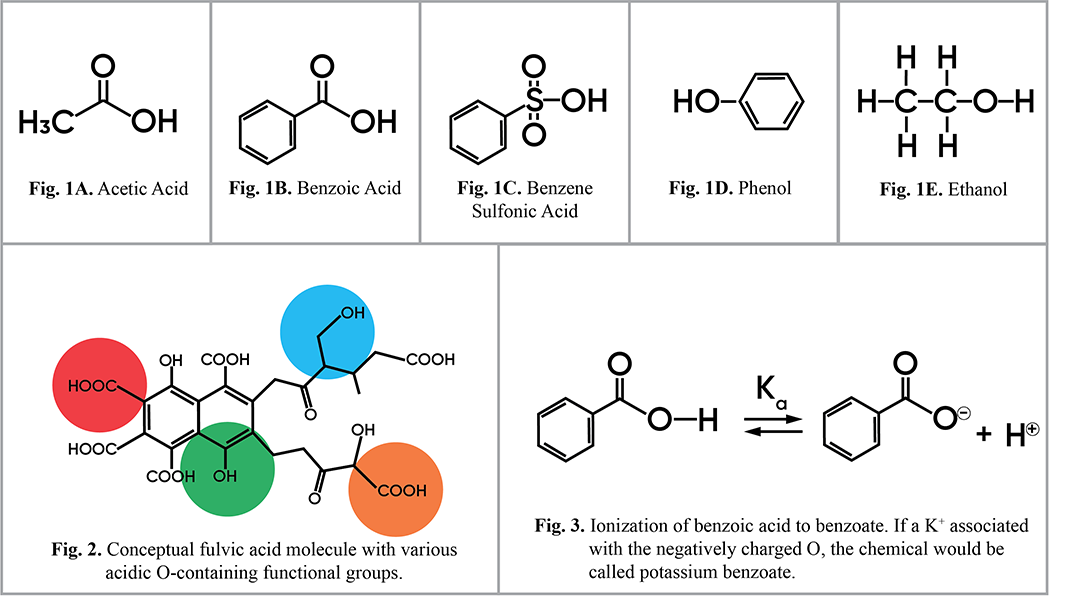
Organic acids are generally weak acids that do not completely dissociate (i.e., donate a proton) in water in the way that strong mineral acids do, such as in the case of hydrochloric acid (HCl). The most common organic acids are carboxylic acids, sulfonic acids, phenols and alcohols (Figure 1).
Organic acids can be aliphatic (structured as open chains rather than aromatic rings), such as acetic acid (Fig. 1A) or ethanol (Fig. 1E). Organic acids can also be aromatic (made up of ring structures, originally named so because of their fragrant properties), such as benzoic acid (Fig. 1B), benzene sulfonic acid (Fig. 1C) or phenol (Fig. 1D).
All of these structures can be found in humic and fulvic acids, sometimes all in the same molecule. For example, one humic acid or fulvic acid molecule might contain a benzoic acid, a phenol, an alcohol, and an aliphatic carboxylic acid (Figure 2). All of these functional groups can ionize (i.e., lose their H+ atoms and contribute to acidity) (Figure 3). The primary factor affecting ionization of organic acids is pH.
Figures 1–3. Chemical structures found in organic acids (click on the image)
We will discuss the interrelationship of soil, pH, and humic substances in Humic Corner #4.
Related Posts

Wastewater Wednesday: Swansea in Massachusetts Uses Federal Funding for Sewer Expansion

Microplex® JS Jump Starts Utah Summer Camp WWTF
by Heather Jennings, PE If I had to choose a favorite of our microbial products it would have to be our Microplex® JS product. It is a two-part formulation of a live synergistic blend of natural, Class I bacteria, specifically chosen for their ability to rapidly degrade solids, fats, lipids, proteins, detergents, hydrocarbons, and other

BHN Article on Micronutrients in CropLife Magazine
CropLife Magazine currently features an article written by BHN staff, “Micronutrients Are the Key To Better Yields.” The authors provide an overview of micronutrients and their relationship to the soil, common deficiencies, application methods, and the importance of following the 4Rs of Nutrient Stewardship. The article concludes by discussing the importance of developing a Micronutrient Plan